Abstract
Background: Limited data exist regarding histological transformation in patients with Waldenström macroglobulinemia (WM). In this study, we present the disease characteristics, outcomes and risk factors for histological transformation in WM.
Methods: Patients with WM seen at Mayo Clinic, Rochester, MN between January 1996 and December 2017 were included. Patients with aggressive non-Hodgkin lymphoma in the setting of WM were considered to have transformation. Univariate analysis for comparing baseline characteristics of transformed versus non-transformed WM was performed using Wilcoxon, Fisher's exact and Chi square tests, as appropriate. Multivariate analysis was performed using logistic regression analysis. Cox proportional hazard method was used to assess the impact on time-to-transformation. All time-to-event analyses were calculated by the Kaplan-Meier method.
Results: Of 1014 patients with WM, 42 patients (4.1%) developed histological transformation. The median follow-up for the entire cohort was 9.5 years (95% CI: 8.8-10.5 years). The cumulative probability of transformation was 2.3% at 5 years, 5.3% at 10 years and 8.5% at 15 years from diagnosis of WM, respectively. There was no difference in the 5-year cumulative probability of transformation in patients with WM diagnosed between 1996-2000 (2.1%), 2001-2005 (2.9%), 2006-2010 (2.7%) and 2011-2015 (3.8%), p=0.61.The disease characteristics, laboratory parameters and histology at transformation are outlined in Table 1. In patients with DLBCL histology (n=39), Revised-International Prognostic Index was calculable in 28 patients, with 50% (n=14), 46% (n=13) and 4% (n=1) of DLBCL belonging to poor, good and very good risk groups, respectively. For DLBCL histology, 18 patients were classifiable using Hans algorithm into germinal center B-cell (GCB) type (n=3, 17%) and non-GCB type histology (n=15, 83%). The median lines of therapy received prior to transformation for the entire cohort was 2 (range 0-9), with 5 (11%) patients not having received any therapy for WM before transformation. Number of lines of alkylator-based therapy used prior to transformation [median 1 line (range 0-4)] was comparable to that used in patients in whom WM did not occur [median 1, (range 0-6); p=0.78].
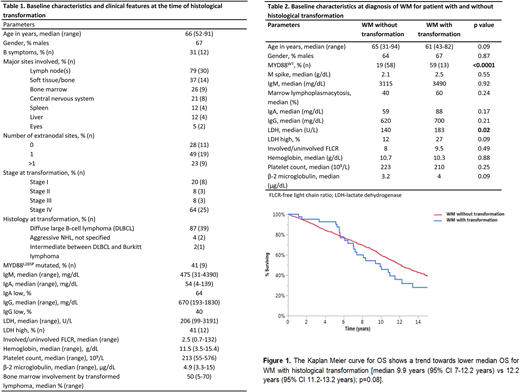
The median time-to-transformation from diagnosis of WM was 4.8 years (95% CI: 2.6- 6.8 years). Overall survival (OS) from transformed disease was 3.2 years (95% CI 1.1-3.9 years). The 10-year OS of patients with WM who transformed was lower (46%) compared to patients without transformation (60%) with a trend towards statistical significance (p=0.08), Figure 1. The MYD88L265P mutation status was available in 333 patients in the entire cohort (22/42 pts in the transformed cohort and 311/972 in the non-transformed cohort. Of the 22 patients in the transformed WM cohort, 13 (59%) exhibited MYD88WT genotype and 9 (41%) exhibited MYD88L265P genotype. The risk of transformation was higher in patients with MYD88WT status [Odds ratio 6.3 (95% CI: 2.6-15.5); p<0.0001]. Additionally, serum lactate dehydrogenase (LDH) was higher at diagnosis of WM in patients who transformed, Table 2. On multivariate analysis, MYD88WT status remained an independent predictor of disease transformation [p=0.003; odds ratio 10 (95% CI: 2.1-48)]. On univariate Cox proportional hazard analysis for identifying predictors of time-to-transformation, only MYD88WT mutational status was associated with shorter time-to-transformation with a 5-year transformation rate of 15.5 % for MYD88WT versus 2.6 % with MYDL265P mutated patients [risk ratio 6.2 (95% CI: 2.5-16.4); p<0.0001]. Treatment-related details in the transformed cases were available for 37 (90%) patients; R-CHOP was the most common (41%) frontline therapy for transformation; 33% patients underwent autologous stem cell transplantation for transformed disease during their disease course.
Conclusion: Histological transformation is an uncommon event in WM but confers poorer survival. MYD88WT genotype is independently associated with histological transformation a shorter time-to- transformation in patients with WM.
Ansell:Trillium: Research Funding; Pfizer: Research Funding; LAM Therapeutics: Research Funding; Seattle Genetics: Research Funding; Affimed: Research Funding; Takeda: Research Funding; Celldex: Research Funding; Regeneron: Research Funding; Merck & Co: Research Funding; Bristol-Myers Squibb: Research Funding. Gertz:Apellis: Consultancy; Alnylam: Honoraria; spectrum: Consultancy, Honoraria; janssen: Consultancy; annexon: Consultancy; Prothena: Honoraria; Abbvie: Consultancy; Amgen: Consultancy; celgene: Consultancy; Teva: Consultancy; Medscape: Consultancy; Ionis: Honoraria; Research to Practice: Consultancy; Physicians Education Resource: Consultancy. Dingli:Millennium Takeda: Research Funding; Millennium Takeda: Research Funding; Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.; Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.. Witzig:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Dispenzieri:Celgene, Takeda, Prothena, Jannsen, Pfizer, Alnylam, GSK: Research Funding. Lacy:Celgene: Research Funding. Kumar:Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kapoor:Takeda: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal